LAHORE - Call it apathy or lust for money, a multinational pharmaceutical company is selling a life-saving drug at three times higher rate than the import price.
The most disturbing aspect of the issue is that the influential pharmaceutical company has been doing this business for years with the approval of the concerned ministry which has allowed it to sell the drug at the exorbitant rate.
Since it has got official approval from the Drug Regulatory Authority of Pakistan (DRAP), the sale at higher rates is officially ‘not illegal’.
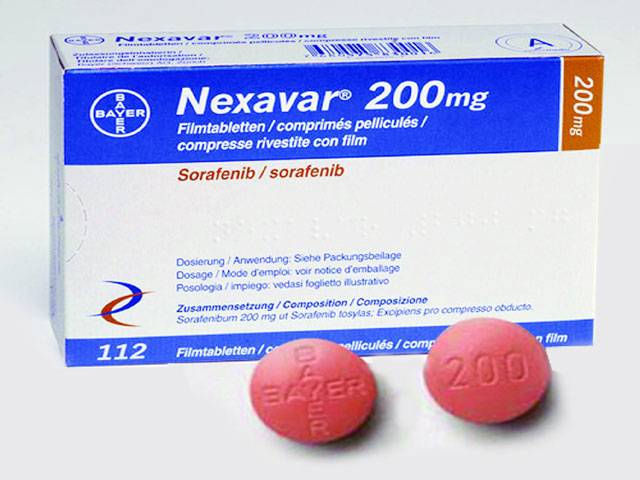
Official documents reveal that Bayer, a noted pharmaceutical company, imports Nexavar (Sorafenib) tablets meant for treatment of liver, kidney, lungs and brain cancers and advanced thyroid carcinoma, at Rs76,687 (60 tablets for 15 days therapy) and sells the same at Rs232,800, around three times higher than the import price.
The patients here have no other option but to buy this imported drug from the market at higher price since the product is not manufactured locally by any of the pharmaceutical companies.
In a sharp contrast to the situation in Pakistan, the neighbouring India is manufacturing the same drug locally which is available in their marker at Rs10,000 only.
An official has pointed out this anomaly in a letter addressed to the Secretary National Health Services, Regulations and Coordination pleading that the price may be rationalised.
Dr Obaid Ali, Federal Inspector of Drugs, Karachi, has further asked the federal secretary to constitute a committee to probe as to how the pharmaceutical company in question managed to get such a high rate approved for a life-saving drug from the DRAP.
Dr Obaid Ali also conveyed to the federal secretary that Bayer neither performs any test on imported drugs nor does it retain any representative sample to investigate any complaint regarding quality, safety and efficacy of products.
He pointed out that this drug is manufactured in India in a facility producing medicines for the US and the UK, and is sold at Rs 10,000/pack.
Sorafenib’s normal dosage is 800mg daily i.e. 4 tablets (two in the morning and two in the evening). Treatment continues till patient is cured or drug starts giving no response to cancer.
President Young Pharmacists Association Dr Nabila Latif said that DRAP has left cancer patients at the mercy of profiteers.
She lamented that the regulators have never facilitated local pharmaceutical companies to manufacture Sorafenib on economical price like in India.
If facilitated, she said, Pakistani companies can manufacture and provide these medicines even at lesser cost than in India.
“We demand the government to facilitate young pharmacists to develop manufacturing facilities. We will not only manufacture cost-effective medicines for local use but will also earn huge foreign exchange by exporting the best quality drugs around the globe,” she said.
Sunday, May 18, 2025
Life-saving drug: imported at Rs76,687, sold at Rs232,800
Cancer patients left at mercy of profiteers as price has DRAP’s approval
-
Lahore emerges among safest global cities in Numbeo 2025 index
-
Lahore emerges among safest global cities in Numbeo 2025 index
-
India’s suspension of Indus Water Treaty legally baseless
-
Seventh polio case reported in Pakistan amid nationwide vaccination drive
-
Pakistan reports sixth polio case of 2025
-
PTA begins issuing VPN licences to regulate usage
Culture Shift
May 18, 2025
Tactical Shift
May 18, 2025
Unmasked Cruelty
May 18, 2025
India Isolated
May 17, 2025
Descent into Hell
May 17, 2025
Fostering Entrepreneurship
May 18, 2025
Behind a Promising Job Offer
May 18, 2025
Indo-Pak Wisdom
May 18, 2025
Traffic Signal at Shahzad Town
May 18, 2025
English a Measure of Intelligence?
May 17, 2025
ePaper - Nawaiwaqt
Nawaiwaqt Group | Copyright © 2025





